|
||||||||||||
|
|
|
The Carbon Cycle
Carbon is exchanged, or "cycled" among Earth's oceans, atmosphere, ecosystem, and geosphere. All living organisms are built of carbon compounds. It is the fundamental building block of life and an important component of many chemical processes. It is present in the atmosphere primarily as carbon dioxide (CO2), but also as other less abundant but climatically significant gases, such as methane (CH4).
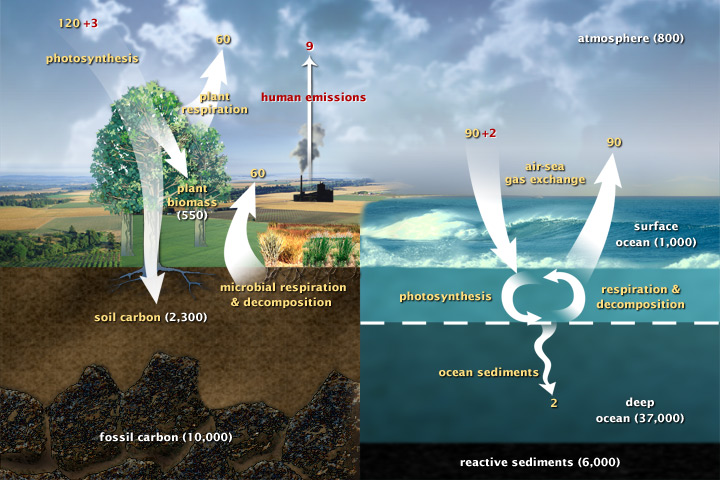
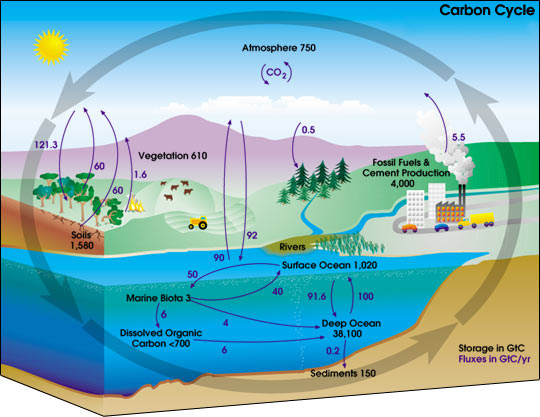
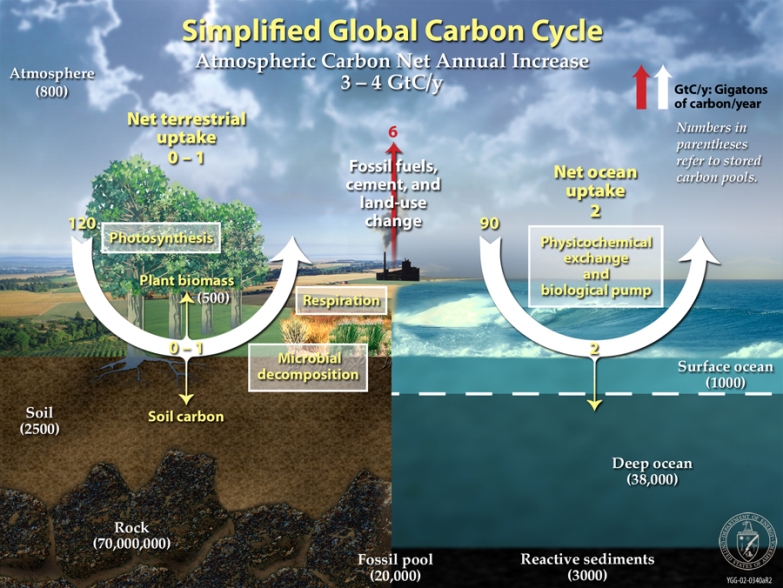
This diagram of the fast carbon cycle shows the movement of carbon between land, atmosphere, and oceans. Yellow numbers are natural fluxes, and red are human contributions in gigatons of carbon per year. White numbers indicate stored carbon. (Diagram adapted from U.S. DOE, Biological and Environmental Research Information System.) Sources and Sinks Because life processes are fueled by carbon compounds which are oxidized to CO2, the latter is exhaled by all animals and plants. Conversely, CO2 is assimilated by plants during photosynthesis to build new carbon compounds. CO2 is produced by the burning of fossil fuels, which derive from the preserved products of ancient photosynthesis. The atmophere exhanges CO2 continuously with the oceans. Regions or processes that predominatly produce CO2 are called sources of atmospheric CO2, while those that absorb CO2 are called sinks. CO2 in the atmosphere acts like a blanket over the planet by trapping longwave radiation, which would otherwise radiate heat away from the planet. As the amount of CO2 increases, so will its warming effect. CO2 is the largest contributor (currently 63%) to this effect by long-lived gases and its role increases each year. The additional burden of CO2 in the atmosphere will remain for a very long time, of the order of thousands of years, if we have to rely on the natural mechanisms of erosion and sedimentation to process the added CO2.
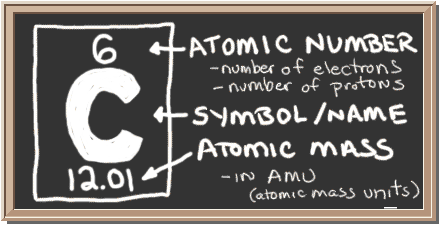
Carbon (C), the fourth most abundant element in the Universe, after hydrogen (H), helium (He), and oxygen (O), is the building block of life.
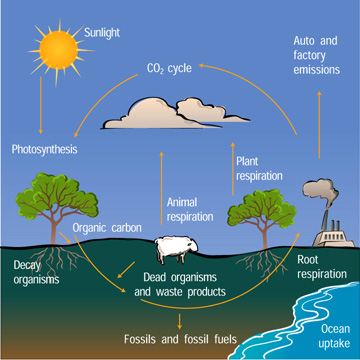
It’s the element that anchors all organic substances, from fossil fuels to DNA. On Earth, carbon cycles through the land, ocean, atmosphere, and the Earth’s interior in a major biogeochemical cycle (the circulation of chemical components through the biosphere from or to the lithosphere, atmosphere, and hydrosphere). The global carbon cycle can be divided into two categories: the geological, which operates over large time scales (millions of years), and the biological/physical, which operates at shorter time scales (days to thousands of years).
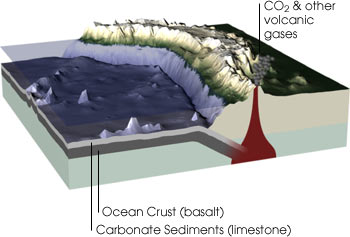
Carbon is the essential element for life on Earth. Not only is carbon found in all living things, the element is present in the atmosphere, in the layers of limestone sediment on the ocean floor, and in fossil fuels like coal. (Illustration by Robert Simmon, NASA GSFC) Geological Carbon Cycle Billions of years ago, as planetesimals (small bodies that formed from the solar nebula) and carbon-containing meteorites bombarded our planet’s surface, the carbon content of the solid Earth steadily increased. Since those times, carbonic acid (a weak acid derived from the reaction between atmospheric carbon dioxide [CO2] and water) has slowly but continuously combined with calcium and magnesium in the Earth’s crust to form insoluble carbonates (carbon-containing chemical compounds) through a process called weathering. Then, through the process of erosion, the carbonates are washed into the ocean and eventually settle to the bottom. The cycle continues as these materials are drawn into Earth’s mantle by subduction (a process in which one lithospheric plate descends beneath another, often as a result of folding or faulting) at the edges of continental plates. The carbon is then returned to the atmosphere as carbon dioxide during volcanic eruptions.
In the geological carbon cycle, carbon moves between rocks and minerals, seawater, and the atmosphere. Carbon dioxide in the atmosphere reacts with some minerals to form the mineral calcium carbonate (limestone). This mineral is then dissolved by rainwater and carried to the oceans. Once there, it can precipitate out of the ocean water, forming layers of sediment on the sea floor. As the Earth’s plates move, through the processes of plate tectonics, these sediments are subducted underneath the continents. Under the great heat and pressure far below the Earth’s surface, the limestone melts and reacts with other minerals, releasing carbon dioxide. The carbon dioxide is then re-emitted into the atmosphere through volcanic eruptions. (Illustration by Robert Simmon, NASA GSFC)
In any given year, tens of billions of tons of carbon move between the atmosphere, hydrosphere, and geosphere. Human activities add about 5.5 billion tons per year of carbon dioxide to the atmosphere. The illustration above shows total amounts of stored carbon in black, and annual carbon fluxes in purple. (Illustration courtesy NASA Earth Science Enterprise)
The balance between weathering, subduction, and volcanism controls atmospheric carbon dioxide concentrations over time periods of hundreds of millions of years. The oldest geologic sediments suggest that, before life evolved, the concentration of atmospheric carbon dioxide may have been one-hundred times that of the present, providing a substantial greenhouse effect during a time of low solar output. On the other hand, ice core samples taken in Antarctica and Greenland have led scientists to hypothesize that carbon dioxide concentrations during the last ice age (20,000 years ago) were only half of what they are today. The Human Role
Credit: NASA, NOAA, EPA,UCAR |